KeriCure liquid bandage inventor Kerriann Greenhalgh was working on her Ph.D. in organic chemistry at the University of South Florida about a decade ago when her boyfriend (now husband) got a small, awkwardly placed cut between his thumb and index finger.
Traditional bandages wouldn’t stay put, and the liquid bandages on the market at the time lacked the elasticity to move with skin, easily cracking off. Despite the couple’s best efforts, the worst-case scenario happened.
“He ended up with this horrible MRSA infection and almost lost his hand from a small cut,” Greenhalgh says. “I [thought], well, I could have fixed that.”
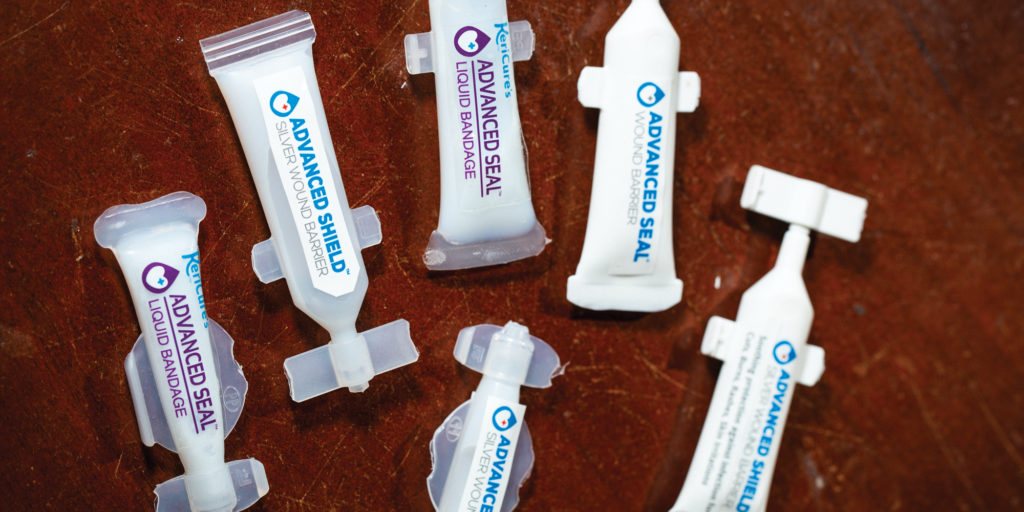
In 2012, Greenhalgh launched KeriCure, a line of liquid bandages made from just water and the company’s proprietary, all-natural polymer. Greenhalgh helped develop the polymer during her time at USF. After graduating with her Ph.D., Greenhalgh licensed the patented technology from USF and developed a flexible liquid bandage that protects cuts, abrasions and other wounds from germs and dirt, while also soothing inflammation and irritation and keeping the skin moist.

“The polymer that’s in our system… mirrors the properties of the skin. It stretches and moves with the body, so you never get that [cracking of the liquid bandage], which is what caused my husband to end up having this infection,” Greenhalgh says. “That was kind of my ‘aha’ moment to start KeriCure.”
Greenhalgh has a lab set up behind her Wesley Chapel home, where she and a small team do the initial research and development of new KeriCure products and formulations. It takes about two years to develop a new product — like their latest, a liquid bandage with natural nanosilver that actively fights bacteria — a process that includes market research, large-scale production at a Tampa manufacturing facility and FDA testing.
KeriCure has already made breakthroughs in wound care. According to Greenhalgh, the company’s Advanced Seal product, targeted at medical professionals, is the first and only liquid bandage cleared by the FDA for use over excisions and incisions. A number of local medispas and dermatologists have successfully used the product over the past two years, and KeriCure will officially launch Advanced Seal to the wider market at the American Academy of Dermatologists’ national meeting at the end of July.
Like with most startups, KeriCure’s biggest challenge has been finding investors willing to share in the vision. Greenhalgh says it’s even harder for biotech companies based in places like Florida — far from the major biotech hubs of Boston and San Francisco — to get funding for clinical trials and business development from venture capitalists and pharmaceutical companies.
“We have some phenomenal biotech coming out of our Florida colleges, and we don’t have any [biotech] industry support. [Inventors] make this great technology, the universities are amazing at getting patents for it, and then it sits,” Greenhalgh says.
Earlier this year, KeriCure was chosen to participate in Quake Capital’s 12-week accelerator in New York. The experience allowed Greenhalgh to make valuable new connections with industry leaders who understand KeriCure’s value lies in its market potential to larger companies, not necessarily the amount of product it’s currently selling.
“We’re not going to get acquired by a big wound care company because we had $50,000 in sales in Publix,” she says. “A company will see [the KeriCure] technology in their pipeline, and they know what the market size is and the movement they can make. We’re just doing proof of concept. KeriCure’s value is based on the technology and the potential within the market.”
Greenhalgh is clear that acquisition is her ultimate goal for KeriCure. She’s a scientist at heart; she says she can be doing the most good developing new products, not hawking them.
“That’s always where our core competency is — in new product development, new formulations, testing and then getting FDA clearance,” Greenhalgh says.
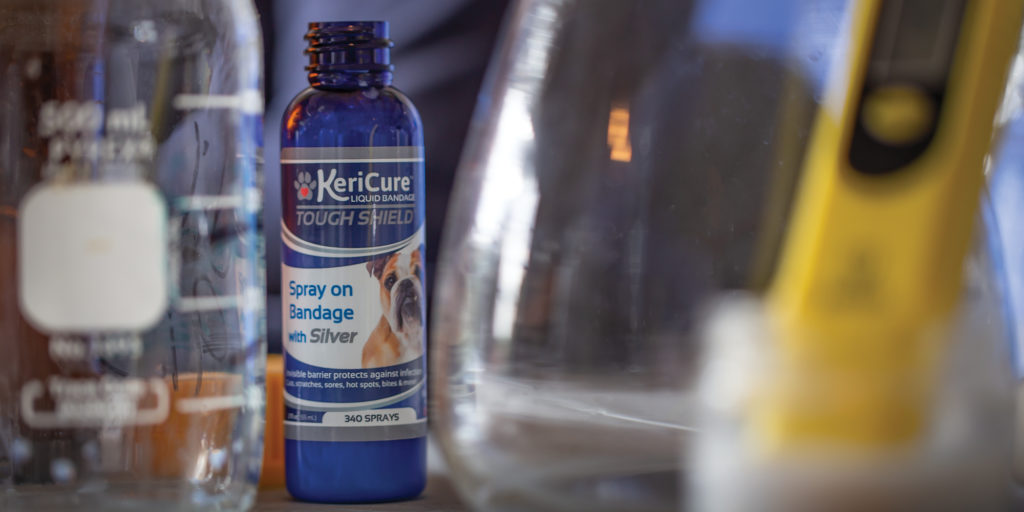
KeriCure is in the midst of a late-stage fundraising campaign, with about a third of its $2 million goal committed already. Greenhalgh says the money will go toward new clinical trials examining the products’ impact on healing times and how well they reduce the irritation of wounds; new products, like the liquid bandage with silver (which is already available for pets and will soon launch for humans on Amazon); and new applications of the product, like elderly care.
Greenhalgh sees the latter as an exciting new market for KeriCure. As we age, she explains, the skin become thin and fragile. When you bandage a cut on an elderly person, removing the bandage often means removing skin as well, creating a tear and a whole new problem. Using a KeriCure product would eliminate the bandage step and the skin tear.
“I feel really strongly that we can help a lot of people [in elderly care],” she says.
KeriCure was based on a simple premise, Greenhalgh says, but it’s one that drives everything the company does: when you have a cut, you need to keep germs out and keep the skin moist.
“That’s all you need,” she says. “So many wound care products try to throw in this and that [ingredient], but we say, keep it simple.”



